How to draw lewis dot structure
Table of Contents
Table of Contents
If you’re studying chemistry, you’ve likely come across the concept of Lewis Dot Structures. But how do you draw them? This guide will take you through the steps to mastering this important skill.
The Pain Points of Drawing Lewis Dot Structures
Many students find drawing Lewis Dot Structures to be a challenging task. It can be difficult to know where to start and how to properly represent the atoms and electrons involved. Additionally, mistakes in a Lewis Dot Structure can lead to incorrect information about the molecule or compound being studied.
The Answer: How to Draw Lewis Dot Structures
First, identify the elements involved and determine how many valence electrons each element has. Next, draw the symbol for the element, surrounded by dots to represent the valence electrons. Remember that each side of the symbol can hold a maximum of two dots before doubling up.
Main Points of Drawing Lewis Dot Structures
When drawing Lewis Dot Structures, it’s important to keep in mind the number of valence electrons for each element, as well as the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell of eight electrons. This will help you properly represent the electrons involved and avoid mistakes.
My Personal Experience with Drawing Lewis Dot Structures
When I was first introduced to Lewis Dot Structures, I found the concept to be overwhelming. However, with practice and guidance from my chemistry teacher, I was able to master the skill. Now, drawing Lewis Dot Structures comes easily to me and I appreciate the insight they provide into the compounds and molecules we study.
 How to Draw Double and Triple Bonds
How to Draw Double and Triple Bonds
When a bond between two atoms involves two or three electrons, respectively, it is called a double or triple bond. To represent this in a Lewis Dot Structure, draw two or three lines between the atoms instead of just one. Then, add the appropriate number of valence electrons to each atom to maintain the octet rule.
 ### Practice Makes Perfect
### Practice Makes Perfect
One of the best ways to master drawing Lewis Dot Structures is to practice, practice, practice. Try drawing structures for different compounds and molecules, and check your work against reliable sources to ensure accuracy. The more you practice, the more comfortable and confident you will become with the skill.
 Common Mistakes to Avoid
Common Mistakes to Avoid
One of the biggest mistakes students make when drawing Lewis Dot Structures is failing to properly account for all valence electrons in the structure. Remember that each valence electron must be accounted for in order to properly represent the molecule or compound. Additionally, be sure to follow the octet rule and avoid exceeding the maximum number of electrons for each atom represented.
Conclusion of How to Draw Lewis Dot Structures
Drawing Lewis Dot Structures can seem daunting at first, but with practice and attention to detail, anyone can master the skill. By properly representing the valence electrons in a molecule or compound, Lewis Dot Structures provide important insight into the properties and behavior of these substances.
Question and Answer
Q: How do I determine the number of valence electrons for an element?
A: The number of valence electrons for an element is typically equal to its group number on the periodic table.
Q: What is the purpose of drawing Lewis Dot Structures?
A: Lewis Dot Structures provide important information about the properties and behavior of molecules and compounds, including their bonding and electron arrangement.
Q: Can molecules have incomplete octets?
A: Yes, some molecules can have incomplete octets, particularly those containing atoms in period three or higher that can exceed eight valence electrons.
Q: How can I check my Lewis Dot Structure for accuracy?
A: Check your work against reliable sources or ask a peer or teacher to review your structure and provide feedback.
Gallery
A Simple Guide For Learning How To Draw Lewis Dot Structures. Use This

Photo Credit by: bing.com / lewis structure dot draw chemistry molecular structures infographic notes organic worksheet classroom help simple drawing diagram electron geometry worksheets chemical
LEWIS DOT DIAGRAM - Unmasa Dalha

Photo Credit by: bing.com / dot lewis diagram structures diagrams chemistry bohr rutherford
3 Ways To Draw Lewis Dot Structures - WikiHow

Photo Credit by: bing.com / wikihow
How To Draw Lewis Dot Structure
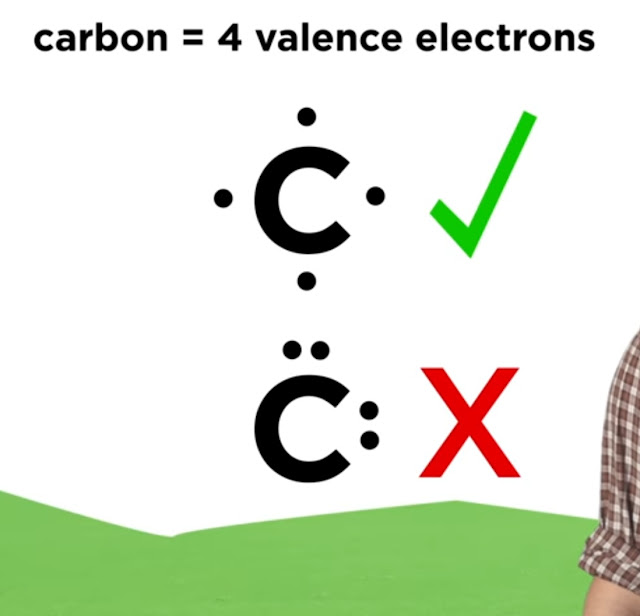
Photo Credit by: bing.com / lewis electron ccl4 electrons valence oxygen
3 Manières De Dessiner Une Représentation De Lewis

Photo Credit by: bing.com /